Key Takeaways
- Actinium is notoriously difficult to study and only available in small amounts. Researchers bound actinium with both a metal-trapping molecule and a protein to grow a crystal and reveal the compound’s atomic structure.
- Scientists often use non-radioactive lighter elements to predict the chemistry of their radioactive heavier counterparts – and were surprised to find actinium behaved differently than expected based on their observations of its lighter counterpart, lanthanum.
- A better understanding of how actinium interacts with other elements could help improve a promising cancer treatment known as targeted alpha therapy.
The element actinium was first discovered at the turn of the 20th century, but even now, nearly 125 years later, researchers still don’t have a good grasp on the metal’s chemistry. That’s because actinium is only available in extremely small amounts and working with the radioactive material requires special facilities. But to improve emerging cancer treatments using actinium, researchers will need to better understand how the element binds with other molecules.

Researchers grew crystals of a pure actinium compound, seen here through a microscope, to understand how actinium binds to other molecules in a solid. (Credit: Jen Wacker/Berkeley Lab)
In a new study led by the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab), researchers grew crystals containing actinium and studied the compound’s atomic structure. While elements often behave similarly to their lighter cousins on the periodic table, researchers were surprised to find that the actinium behaved differently than predicted by looking at its counterpart, lanthanum.
“There’s a breadth of applications for these elements, from nuclear energy to medicine to national security, but if we don’t know how they behave, that inhibits the progress we can make,” said Jen Wacker, first author of the paper published today in Nature Communications and a chemist at Berkeley Lab. “We’re seeing that this work is necessary to really understand the complexity of these radioactive elements, because in a lot of cases, using their surrogates is not sufficient to understand their chemistry.”

One area of interest is in using an isotope of actinium (actinium-225) in a cancer treatment method called targeted alpha therapy (TAT), which has shown promise in clinical trials. The TAT method uses biological delivery systems such as peptides or antibodies to move the radioactive element to the cancer site. When the actinium decays, it releases energetic particles that travel a short distance, destroying the nearby cancer cells but sparing healthy tissue further away.
“There’s a movement to design better delivery systems to get the actinium to particular cells and keep it there,” said Rebecca Abergel, a UC Berkeley associate professor of nuclear engineering and of chemistry who leads the Heavy Element Chemistry Group at Berkeley Lab. “If we can engineer proteins to bind the actinium with a really high affinity, and either be fused with an antibody or serve as the targeting protein, that would really enable new ways to develop radiopharmaceuticals.”
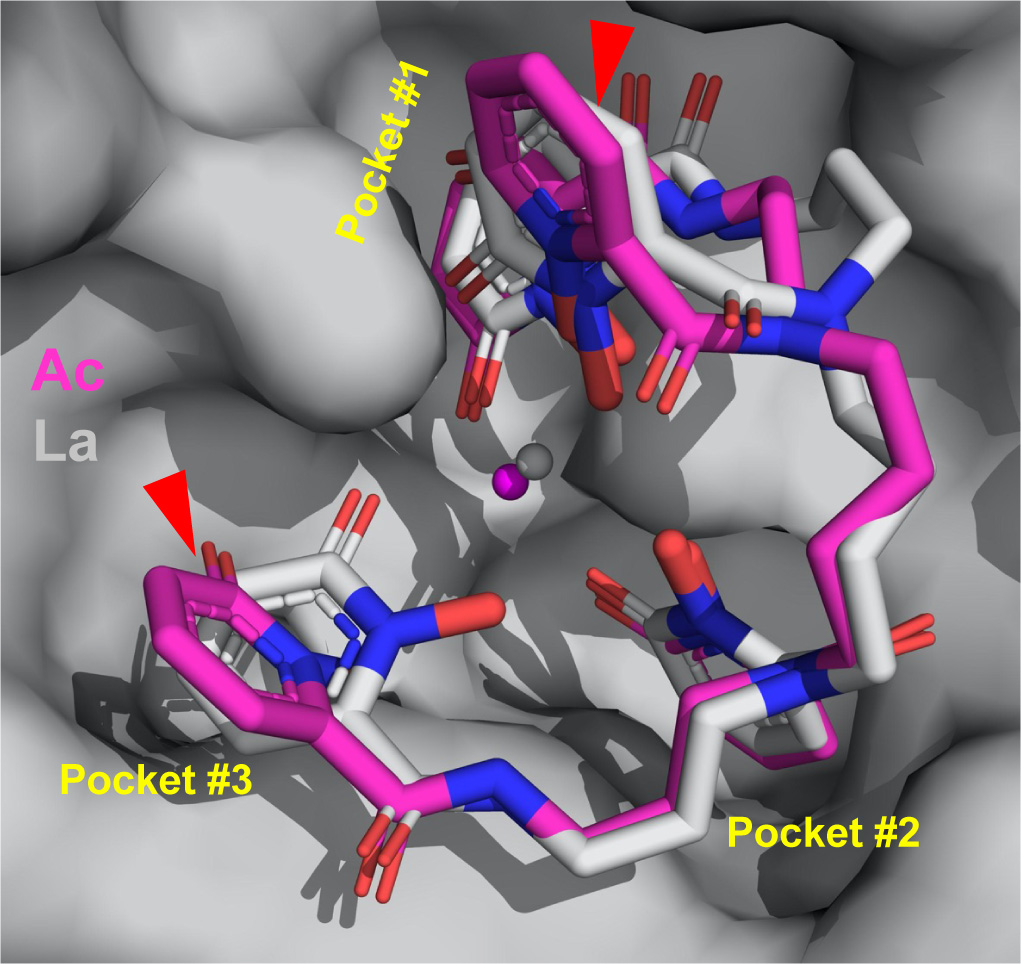
This rendering shows the structure of how actinium (magenta) binds with other molecules. Red triangles point out how the arrangement differs from actinium’s lighter counterpart, lanthanum (grey). The stick structure of the binding molecule (the ligand) is surrounded by pockets in the protein. (Credit: Jen Wacker/Berkeley Lab)
Researchers used a novel approach to grow the crystals using only 5 micrograms of pure actinium – roughly one tenth the weight of a grain of salt, and invisible to the naked eye. They first purified the actinium through a complex filtration process that removed other elements and chemical impurities. They then bound the actinium to a metal-trapping molecule called a ligand and enveloped the bundle inside of a protein isolated and purified by Roland Strong’s team at the Fred Hutchinson Cancer Center, building a “macromolecular scaffold.” The crystals, grown over a week inside of the Heavy Element Research Laboratory, were then cryocooled in liquid nitrogen and illuminated with X-rays at Berkeley Lab’s Advanced Light Source (ALS). The X-rays revealed the compound’s 3D structure and showed how actinium interacted with surrounding atoms. It is the first single-crystal X-ray structure reported for actinium.
“I’ve been working in crystallography for 40 years and seen a lot of things, and the method the team is using is unique and provides details we couldn’t get in the past,” said Marc Allaire, a scientist in Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging Division and head of the Berkeley Center for Structural Biology team at the ALS. “To the best of my knowledge, Berkeley Lab is the only place in the world where we do this kind of study and measure radioactive protein crystals.”
In this work, scientists used actinium-227, the longest-lived isotope of the element. Future studies will explore actinium-225 (the preferred isotope for targeted alpha therapy) to look for other changes in how the metal binds. Researchers are also interested in pairing actinium with different proteins to learn more about the structures it forms.
“This is very fundamental science that is part of our core program in understanding the chemistry of heavy elements,” Abergel said. “We’ve achieved a really technically difficult experimental method that pushes the boundaries of isotope chemistry and lets us gain a better understanding of this element. It hopefully will enable us and others to develop better systems that are useful for targeted alpha therapy.”
The Advanced Light Source is a DOE Office of Science user facility.
###
Lawrence Berkeley National Laboratory (Berkeley Lab) is committed to delivering solutions for humankind through research in clean energy, a healthy planet, and discovery science. Founded in 1931 on the belief that the biggest problems are best addressed by teams, Berkeley Lab and its scientists have been recognized with 16 Nobel Prizes. Researchers from around the world rely on the lab’s world-class scientific facilities for their own pioneering research. Berkeley Lab is a multiprogram national laboratory managed by the University of California for the U.S. Department of Energy’s Office of Science.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit energy.gov/science.



